Insights from a former FDA Medical Officer will help you
prepare for an FDA audit.
Introduction
Clinical Audits
There are a number of reasons for clinical audits. It is important
to:
Define the Audit Objective
Is the Audit:
- Prior to study completion or after study completion?
- To assess the accuracy of monitors or investigator and staff?
- To assess the data for regulatory submission?
- "For cause?"
- Or is this a Pre-FDA Audit? This is to help the site and
company prepare for an FDA Inspection
This poster presentation will offer insights on deficiencies
found by FDA on their inspections and will offer personal experience
from a former FDA medical officer, guidelines for preparing for
an FDA inspection and writing the audit report.
Deficiencies Most Frequently Found by Routine FDA Inspections
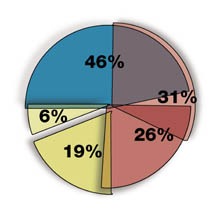 |
- Inadequate Patient Consent Form 46%
- Failure to Adhere to Protocol 31%
- Inadequate and Inaccurate Records 26%
- Inadequate Drug Accountability 19%
- Failure to Keep IRB Informed of Protocol Changes 6%
|
Deficiencies Most Frequently Found by FDA for Cause Inspections
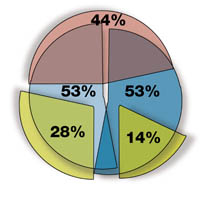 |
- Inadequate Patient Consent Form 44%
- Failure to Adhere to Protocol 53%
- Inadequate and Inaccurate Records 53%
- Inadequate Drug Accountability 28%
- Failure to Keep IRB Informed of Protocol Changes 14%
|
Personal Audit Experience
I would like to share one of my audit experiences.
I audited a company with absolutely no experience with an FDA
audit. The staff had no idea how to prepare. They expected FDA
to focus on the study sites with the problems.
I identified sites which ultimately were audited by FDA. The
company had prepared me for numerous study issues. Actually I
was overwhelmed by issues.
Had FDA walked in as I did, there is no doubt in my mind that
FDA would have left. They would have said, "Let us know
when you are ready for an FDA inspection."
The Regulatory Files were a disaster.
The only thing for certain was that the patients had signed
an Informed Consent. That was a very good sign. There was hope.
Since there was chaos in what should have been Regulatory Documentation,
it was not possible to determine where all the deficiencies were.
There were so many corrections on the CRFs that it was difficult
to determine the correct information when compared to the source documentation.
Before leaving I met with the PI. He had never been through an
FDA audit but was anxious to do well. After my discussions with
him I was convinced that he knew the protocol, had done what
was expected for the study and had obtained IRB approvals.
During our discussions it was clear to me that much of the
original documentation was kept in his private office. With that
information, I was convinced that the product had merit, should
probably be approved and it was my job to help this company get
their act together. My audit report listed deficiencies but more
importantly it gave the company the necessary information to
get focused and prepared for the FDA.
I am pleased to report to you that the site did not get a
483.
Perkins Guidelines For Writing the Audit Report
- Stay Focused - Do not focus on blame
- Focus on Issues - Is the Site ready for the FDA?
- The Report Should Help Prepare the Site for FDA
- Clear Documentation is Essential
- Focus on Solutions
Perkins Guidelines: Preparing for FDA Inspection
- Should the Company conduct the audit or hire outside auditors?
- An outside objective auditor is best.
- What criteria should the company use in hiring an outside
auditor? Experience! Companies should inquire on the experiences
of the auditor.
- Should there be a 100% audit? - Probably not.
- Which sites should be selected for an audit? - Pivotal trial
sites to be used for safety and efficacy
Fraud
The first step to an easy inspection is documentation.
Why is FDA so stubborn on source documentation? FRAUD.
Lets look at a little FDA history. Did you know that clinical
inspections started in the early 1960s with 4 inspectors and
they were all MDs? What they found were the first cases
of fraud.
Look below at 3 of those early fraud cases.
1. Dr. Ernest Brown of Baltimore who admitted that 30 of the
43 charts he submitted on 50 patients were "fabricated."
2. Dr. Kathleen Roberts of San Francisco admitted that the
charts on 57 of 75 patients "were complete fabrications."
3. Dr. Bennett Robin of Silver Spring, MD pleaded "no
contest" to 5 counts of causing pharmaceutical firms to
submit fraudulent clinical results to the FDA.
Although FDA, companies and investigators all worry about
fraud, it is not often found.
Audit regularly. If there is fraud, companies should find
it before FDA.
In Summary
- Always consider an outside objective auditor before
FDA arrives
- Choose an auditor who knows how FDA thinks and how
FDA sees sites
- Prepare your company for the 483
- Work with your auditor and get your site ready for
the FDA
E-Mail Us!